What Is Written First In The Chemical Formula Of A Covalent Molecule
A binary covalent compound is composed of two different elements usually nonmetals. That is the total amount of positive charge must equal the total amount of negative charge The subscripts should be the smallest set of.

Write Chemical Formulas And Names For Ionic And Covalent Compounds And Balance Chemical Equations Ppt Download
Like cations the charge on an anion is indicated using a superscript after a chemical formula.
What is written first in the chemical formula of a covalent molecule. The large exponent means that when R d i then small decreases in R cause large increases in repulsion. Stable molecules exist because covalent bonds hold the atoms together. Short range repulsion only matters when atoms are in very close proximity R d i but at.
Separating any pair of bonded atoms requires energy see Figure 44. The element with the higher group number is written second in the name. The stronger a bond the greater.
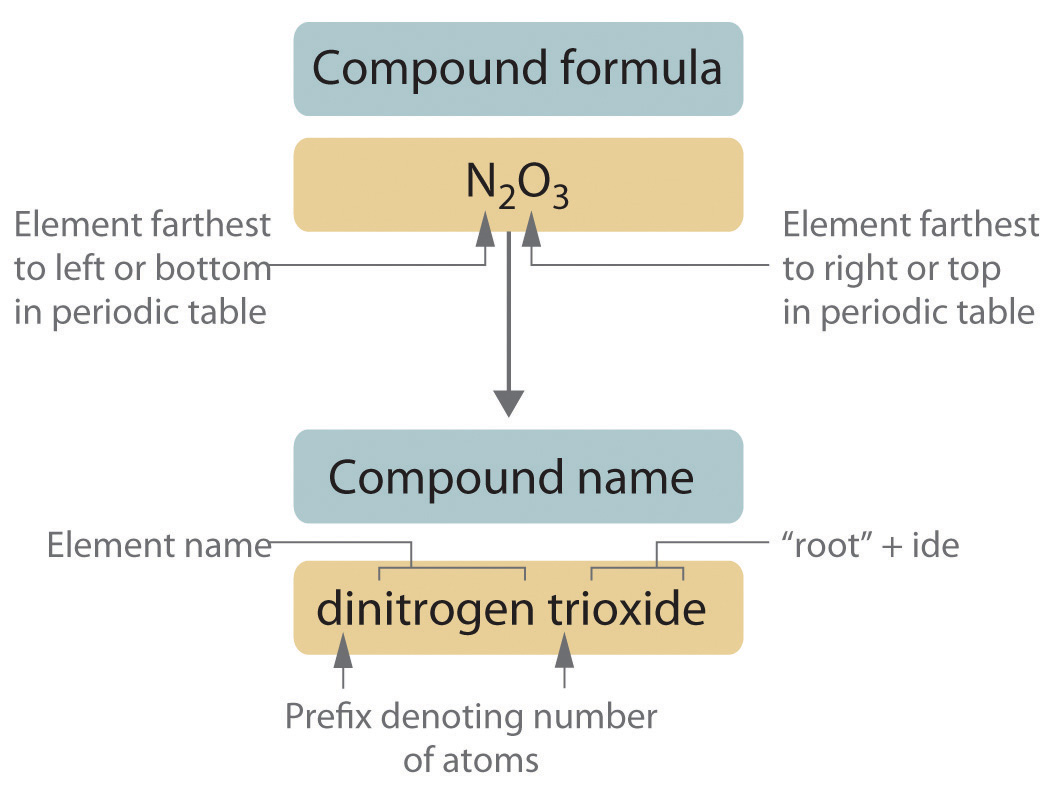
The repulsive energy goes up as d i R 12 where R is the distance between the atoms and d i is the distance threshold below which the energy becomes repulsive. D i depends on the types of atoms. For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine.
We measure the strength of a covalent bond by the energy required to break it that is the energy necessary to separate the bonded atoms. The subscripts in the formula must produce an electrically neutral formula unit. The element with the lower group number is written first in the name.
For example Cl - is the symbol for the chlorine anion which carries a single negative charge -1. Writing the chemical formula of compounds requires identifying chemical symbols understanding numbers in formulas and recognizing key prefixes and suffixes. The cation is written first followed by the monatomic or polyatomic anion.
The number of neutrons is not a factor in whether an atom functional group or molecule is an anion. Prefixes like bi- and tri- help identify the number of ions in a molecule.

4 2 Covalent Compounds Formulas And Names The Basics Of General Organic And Biological Chemistry

Covalent Compounds Writing Chemical Names And Formulas Youtube
Introducing Covalent Bonding Montessori Muddle
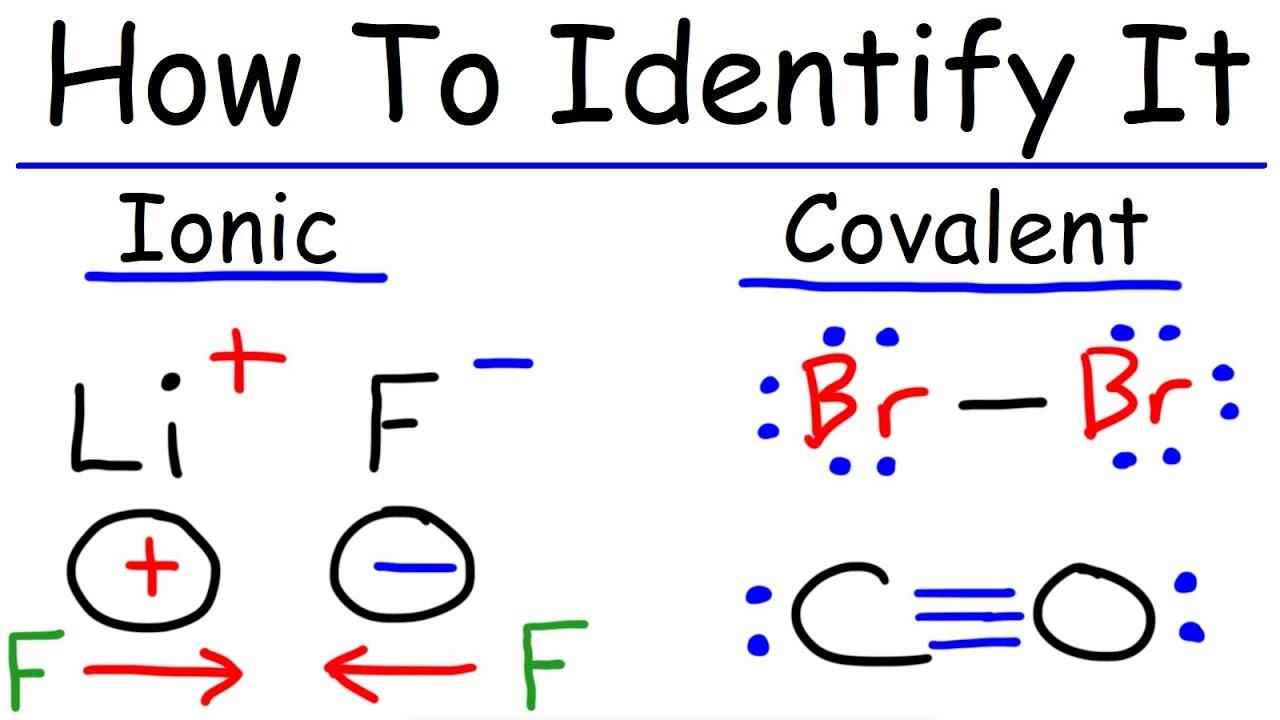
Ionic And Covalent Bonding Chemistry Youtube

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
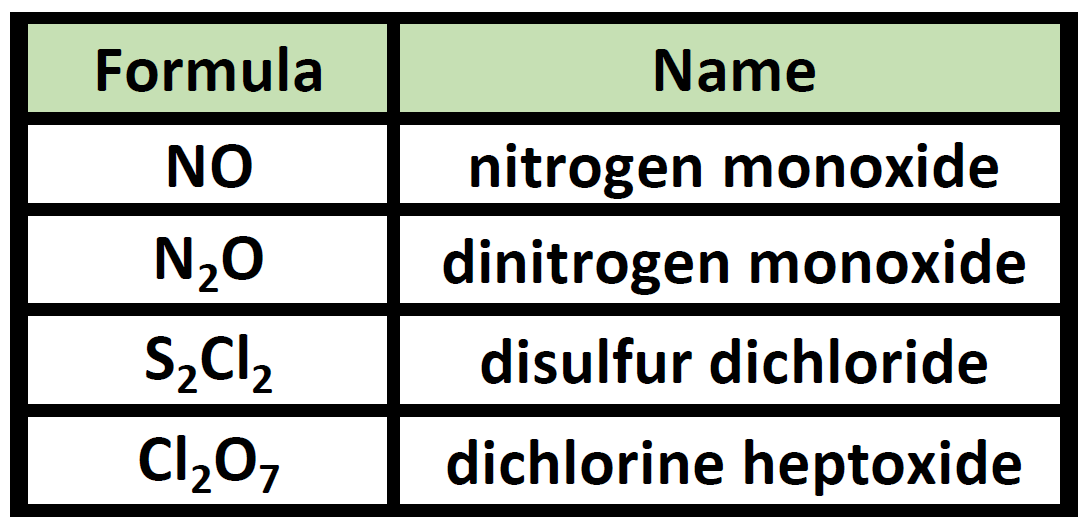
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Molecules Eq How Are The Chemical Formulas And Chemical Names Written For Covalent Molecules Ppt Download

Naming Covalent Compounds Nomenclature Rules

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
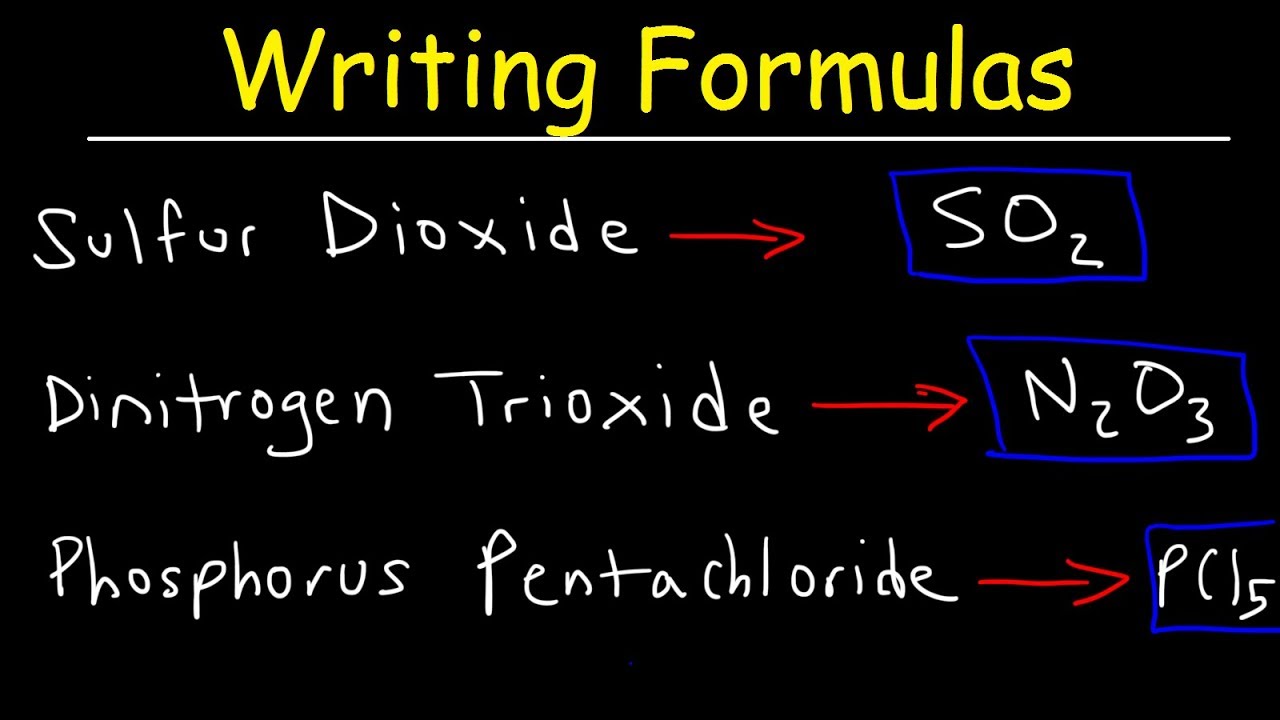
How To Name Covalent Molecular Compounds The Easy Way Youtube
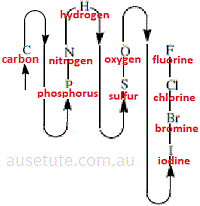
Molecular Formula Of Inorganic Non Metallic Binary Covalent Compounds Chemistry Tutorial
Molecules Ions And Chemical Formulas

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Naming Compounds The Chemical Formula Represents The Composition Of Each Molecule In Writing The Chemical Formula In Almost All Cases The Element Farthest Ppt Download
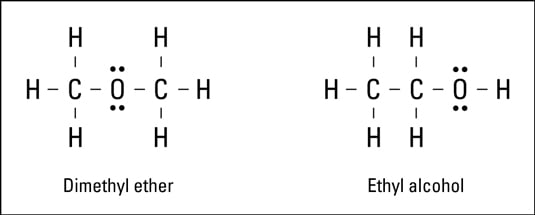
Covalent Bonds Types Of Chemical Formulas Dummies

Covalent Structures Eq How Are The Chemical Formulas And Chemical Names Written For Covalent Molecules How Do You Draw Vsepr Diagrams For Covalent Compounds Ppt Download

How To Write Formulas For Molecular Covalent Compounds

