What Nonpolar Covalent Bonds Are In Glucose
Agents that disrupt hydrophobic interaction. Nonpolar covalent bonds Hydrophobic Insoluble in water Can be identified by the 2 key parts of their assembly.
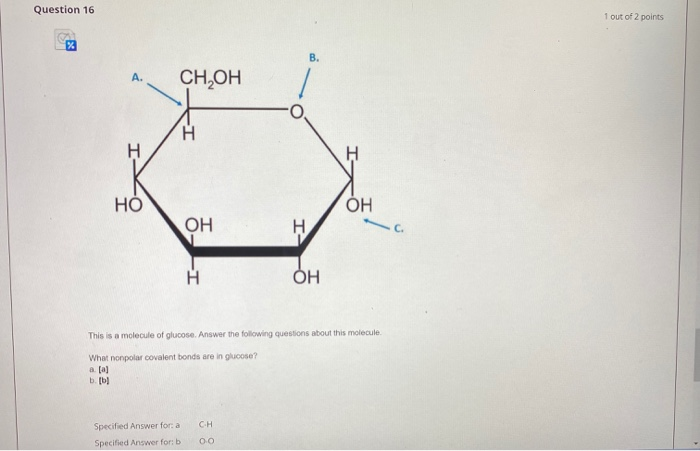
Solved Question 16 1 Out Of 2 Points V A Ch Oh N N No On Chegg Com
Therefore sugars fat and alcohols are nonelectrolytes.

What nonpolar covalent bonds are in glucose. Ionic and covalent bonds are strong bonds that require considerable energy to break. The covalent bonds and functional groups of a bi omolecule are of course central to its function but so also is the arrangement of the molecules constituent atoms in three- dimensional space. Organic solvents such as acetone or ethanol -- dissolve nonpolar groups.
Some compounds such as glucose can dissolve in water but do not ionize. However not all bonds between elements are ionic or covalent bonds. Difference Between Electrolytes and Nonelectrolytes Definition.
The many nonpolar CH bonds in the long hydrocarbon skeleton make fats hydrophobic. The plasma membrane of a cell creates a boundary between a cell and its environment and regulates the molecules that enter and exit the cell. D-glucose is a hell of a lot faster to write and say than 2R3S4R5R23456-pentahydroxyhexanal.
One glycerol backbone and 3 long carbon chains fatty acids Far greater than 21 HO ratio. Typically nonelectrolytes are nonpolar compounds. When protein folding takes place in the aqueous environment of the body the hydrophobic R groups of nonpolar amino acids mostly lie in the interior of the protein while the hydrophilic R groups lie mostly on the outside.
The L-D- system allows for the configuration of a molecule with multiple chiral centers to be summarized with a single letter plus its common name of course. An aqueous solution of glucose is composed of glucose molecules. Hydrogen bonds of the alpha-helix will be replaced by hydrogen bonds to urea for example and the helix will unwind.
These are attractions that occur between positive and negative charges that. Weaker bonds can also form. Fats separate from water because the water molecules hydrogen bond to one another and exclude the fats.
Cysteine side chains form disulfide linkages in the presence of oxygen the only covalent bond forming during protein folding. Detergents -- dissolve nonpolar groups. Examples of nonpolar bonds include methane middle and oxygen right.
B peptide bonds The bonding of two amino acid molecules to form a larger molecule requires a both the release of a carbon dioxide molecule and the addition of a nitrogen atom. In a fat three fatty acids are joined to glycerol by an ester linkage creating a triacylglycerol or triglyceride. Cold -- increases solubility of nonpolar groups in water.
Does Glucose Classify As Polar Or Non Polar Quora

General Chemistry Video Playlist Youtube Element Chemistry Chemistry Activities

Polar Covalent Bonds Covalent Bonding Teaching Chemistry Chemistry Help

What Makes A Good Nucleophile Master Organic Chemistry Organic Chemistry Physics And Mathematics Chemistry

Cellular Respiration Overview Cellular Respiration Cellular Electron Transport Chain
Does Glucose Classify As Polar Or Non Polar Quora
Does Glucose Classify As Polar Or Non Polar Quora

Is Glucose Polar Or Nonpolar C6h12o6 Youtube

Fed Fasted Metabolism Pathways Bioquimica Microbiologia Apuntes De Clase
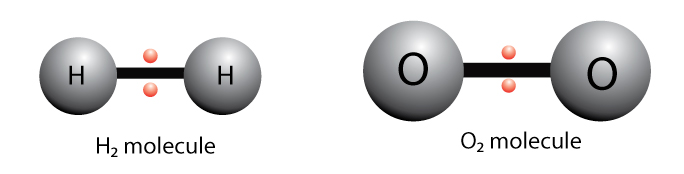
What Is Nonpolar Covalent Bond

Balancing Equations With Skittles Balancing Equations Equations Lib
New Ap General Chemistry Video Playlist Youtube Chemistry Conservation Of Mass Organic Chemistry Tutor

Solved Please Help With How Many Non Polar Covalent Bonds Chegg Com

Mcat Biochemistry Macromolecules Fatty Acids Biochemistry Mcat Macromolecules

Covalent Bonds Biology For Majors I

Essay Fast Food Spm In 2021 Essay Essay Writing Tips Review Essay